Ronovo Surgical Debuts Modular Robotic Platform For Laparoscopy At SRS 2023
MELBOURNE, AUSTRALIA -- Ronovo Surgical recently attended the 13th Annual Meeting of the Society of Robotic Surgery (SRS 2023), which took place from July 24-26 in Melbourne. An innovative Chinese medtech company, Ronovo is pioneering the development of modular surgical robots.
SRS Annual Meeting is one of the world’s premier multidisciplinary robotic surgery conferences, and this year it was jointly hosted by Prof. Vipul Patel from AdventHealth Global Robotics Institute and Prof. Anthony Costello from International Medical Robotics Academy. The meeting this year brought together over a thousand attendees, including robotic surgeons, industry participants, and investors from around the world. The focus was on cutting edge topics in the field of robotic surgery, with discussions and insights into global development of robotic surgery and the future of the surgical robotics industry.
In early June, Prof. Patel visited Ronovo in Shanghai to test-drive the Carina RAS Platform. Impressed by the low learning curve and high performance of the first modular surgical robot in the Chinese market, he encouraged the team to present Carina internationally and extended an invitation to SRS 2023. Just a month later, Ronovo debuted Carina to a global audience and garnered significant attention from industry.
During SRS 2023, Dr. John Ma, Founder and CEO of Ronovo, presented on the company's journey and the current state of the Chinese surgical robotics market. The audience resonated with Dr. Ma’s conviction that “modularity” is the optimal solution to drive widespread adoption of RAS.
Since the completion of China's first laparoscopic robotic surgery in 2007, RAS has undergone 16 years of development in the Chinese market. Currently, Chinese laparoscopic surgeons perform over 10 million minimally invasive surgeries (MIS) annually. However, the clinical penetration rate of RAS in China is less than 1%, with less than 0.5% of minimally invasive surgeons capable of performing RAS. Clearly, the Chinese RAS market holds immense untapped potential.
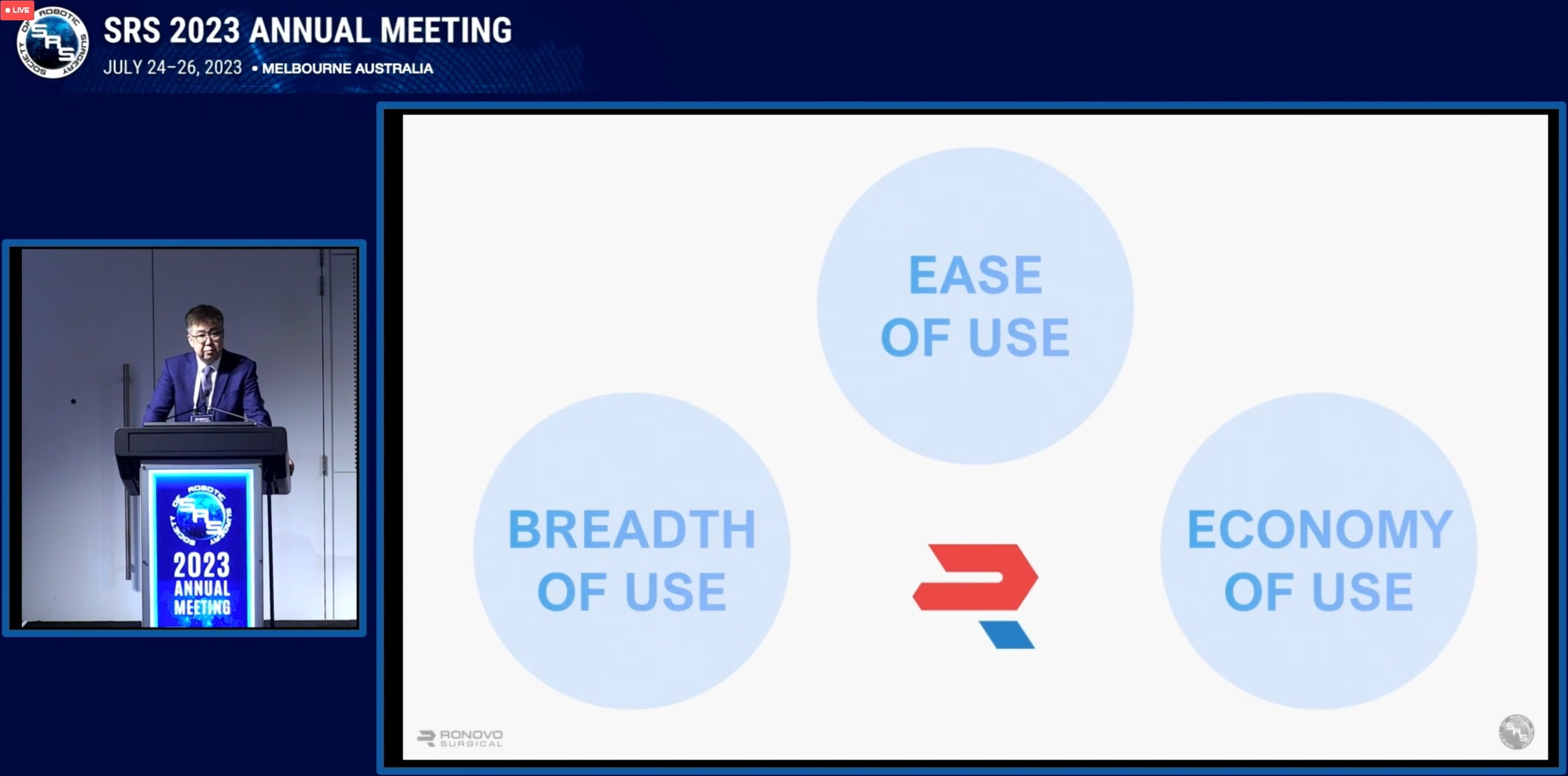
During his presentation on Ronovo, Dr. Ma shared three essential elements for driving the widespread adoption of RAS:
1. Ease of Use: Robotic systems must be simple to learn and easy to use, enabling minimally invasive surgeons to perform RAS more effortlessly.
2. Breadth of Use: Robotic systems should not be limited to urology and must find clinical applications across various surgical specialties, such as gynecology, general surgery, and more.
3. Economy of Use: The economic value of robotic systems is crucial, because hospitals must consider the return on investment when procuring these devices.
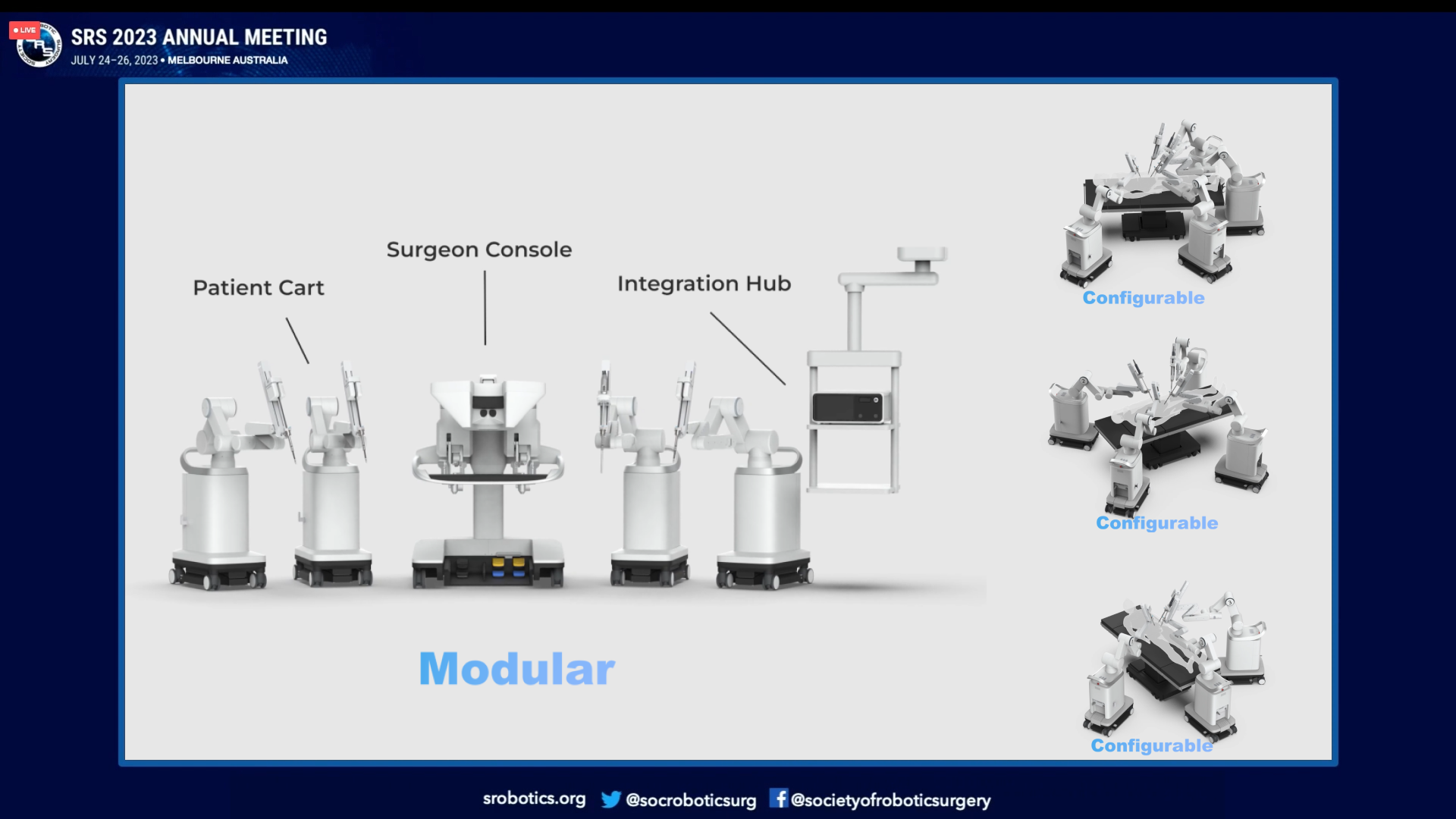
The modular design of Carina aligns well with these three elements. Operating rooms (ORs) in China typically have limited space, with only 33 square meters available., which dictates that robotic systems must be compact. The flexibility of modular design makes Carina an ideal solution. Surgeons may have varying preferences and habits at different stages of their careers, and modular single-arm robotic platforms allow customization based on individual needs. Additionally, compatibility with traditional surgical methods ensures a smooth transition. Given the financial burden on Chinese patients, cost-effectiveness is critical. The inherent flexibility and configurability of modular surgical robots help reduce overall costs.
Ronovo’s founding team addressed numerous clinical challenges by collaborating with clinical experts. Through extensive testing, simulation, trial and error, and validation, they successfully pioneered the path of modular surgical robots in China, developing proprietary core technology. As a result, Ronovo became the first company in China to develop a modular surgical robotics platform.

Voice of customer is highly valued by Ronovo, and its team recognizes the importance of collaborating closely with clinical doctors in the development of large medical capital equipment, especially in the field of surgical robotics. From the inception of the concept design, Ronovo has worked extensively with both domestic and international MIS experts. They have continuously gathered feedback from doctors in various specialties, used this feedback to refine and optimize their system, and iterated rapidly to enhance the user experience and system stability.
Dr. Ying Mao, CTO/COO of Ronovo, emphasized the importance of the "voice of customer" during his presentation at SRS 2023, stating that understanding the clinical needs and workflow of customers, or surgeons in this case, was crucial during the initial concept design of Carina.
In the early stages of R&D, every Ronovo engineer spent time observing RAS in the OR to gain a profound understanding of how surgeons use robotic systems, and this hands-on experience helped identify unmet clinical needs. To truly understand growth barriers in the Chinese RAS market, the Ronovo founding team spent a year collaborating with surgeons from various hospitals and specialties. This extensive effort led to the confirmation of the modular design in 2020.
Ronovo remains committed to integrating clinical feedback into the product design journey. From the initial design reviews to the feedback on components and prototypes, timely guidance from surgeon experts throughout the development process has helped Ronovo optimize its system, validate requirements, and align its product with clinical needs. After undergoing extensive preclinical animal and cadaveric testing and receiving unanimous praise from top surgeons, Carina and its modular design, together with proprietary intellectual property rights, is now approaching human clinical trials.

Prof. Dingwei Ye, a renowned urology oncology expert who presides as Chief of Urology at Fudan University Shanghai Cancer Center and serves as Chief Urology Consultant for Ronovo, has a remarkable track record in RAS, with his team having successfully completed over 8,000 cases of RAS.
During the "New Technologies in Urology: The Future" segment at SRS 2023, Prof. Ye not only shared his decades of experience in RAS but also showcased the clinical application achievements of Carina through his own experiences.
Prof. Ye stated, "Ronovo has left a deep impression on me. Their team has the capability and ambition, and collaborating with them is a challenge for me." He then shared the results of more than ten preclinical animal and cadaveric trials he conducted using Carina. These trials covered a wide range, from partial kidney resections in a pig model to prostatectomies in a dog model to similar procedures on cadaveric model. He provided comprehensive data on various parameters, including preoperative preparations, intraoperative procedures, blood loss, and postoperative conditions, to demonstrate the performance and stability of Carina.
"These successful preclinical trials have preliminarily verified the safety and effectiveness of Carina in urological surgeries. Carina is capable of performing complex and precise surgical procedures to meet the demands of urological surgeries," added Prof. Ye. Further validation of Carina will require more specialized clinical trials, and Prof. Ye will oversee the first human clinical trial of Carina in China following the conclusion of SRS 2023. Stay tuned for more updates on this exciting development.
Contact
ZiHan Lin
VP of Business Development
zihan.lin@ronovosurgical.com






